Atoms tap all the time! But to understand why we must first decide what we mean by the word “touch.”
Our normal concept of touch is based on the macroscopic world. I put a glass on the table – the glass touches the table. You dip your toes in the water – you touch the water, etc. In all of these cases, one solid boundary or surface (the bottom of a glass, the edge of your finger) touches another solid boundary or surface (the top of a table, the surface of the ocean). But our macroscopic concepts break down at the microscopic level, hence the confusion with “touch.”
Read more:Will humans ever be faster than light?
Atoms and boundaries
If we can increase atomic scales, we would see a madhouse. Atoms and molecules are constantly flying around, bumping into each other, twisting, spinning, and generally making a mess. But one thing quickly becomes apparent: atoms have no strict boundaries.
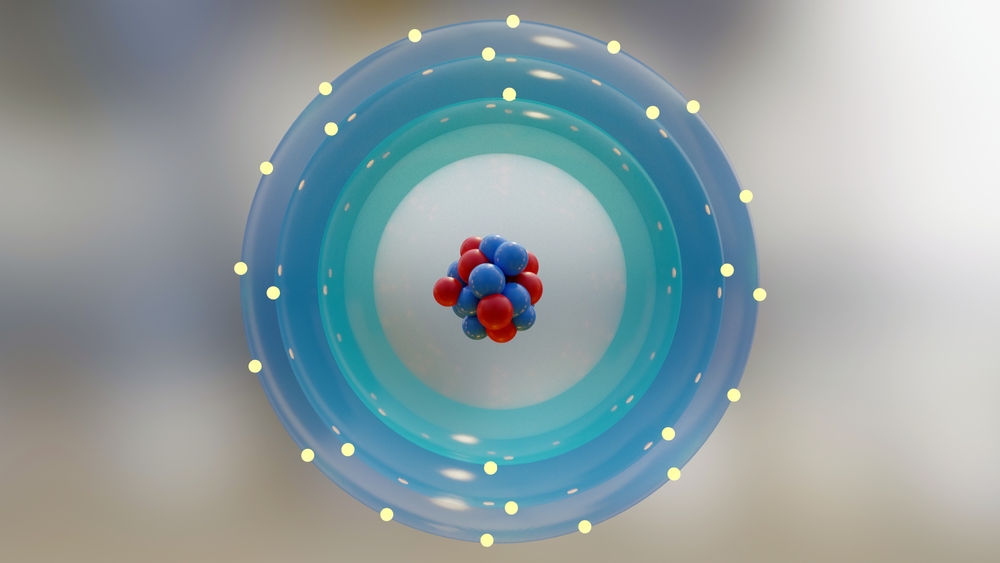
This is the nucleus, a bundle of protons and neutrons at the center, surrounded by clouds of probability of where the orbiting electrons might be the next time we go looking for them. The theory of quantum mechanics tells us how to calculate these probabilities, and the range of these probabilities covers the entire universe. However, almost every time we look at an atom, the electrons are safely bound near the nucleus, so unless we’re doing high-energy collider experiments, we don’t have to worry too much about this.
Since atoms have no solid surface, in a sense there is nothing to “touch” because there is never a situation where one boundary meets another boundary. But “touch” also conveys a sense of close and personal influence, and in that sense atoms touch all the time.
Macroscopic vs. Microscopic
Atoms interact with each other through electromagnetic force, because electrons and protons in atoms are electrically charged. Technically, this force has an infinite range, but it only becomes significant when the atoms are close enough. Sometimes the atoms literally bounce off each other due to the repulsive electromagnetic force between their electrons – in that brief instant it’s hard to describe their interaction as anything other than touching.
Read more: Is there a particle that can travel back in time?
Even if the interaction isn’t brief, it’s still considered a touch. When it comes to this cup on the table, if we zoom in on the individual atoms and molecules, we will see the electrons at the very outer edge of the cup being repelled by the electrons at the very outer edge of the table. Even if there is a gap between the two electron shells (and there almost always is), the atoms are close enough to influence each other significantly. This is evidenced by the fact that the cup does not simply slide through the mass, and that the electromagnetic forces between the atoms are sufficient to counteract the force of gravity pulling the cup toward Earth.
When we say that two objects touch on a macroscopic scale, it means exactly that on a microscopic scale.
Nuclear fusion
Atoms can touch in other ways as the electromagnetic force is not always repulsive. When the atoms get close enough, a manifestation of the electromagnetic force occurs, the so-called van der Waals force, which can cause atoms to bond together. This is exactly how molecules are formed, and the atoms inside molecules definitely touch.
Finally, even the nuclei inside atoms can touch. This is incredibly difficult to do because of the extremely strong electromagnetic repulsion between the positively charged protons in each nucleus. But again, quantum mechanics enters the picture. If two atoms get close enough for long enough, then sometimes, completely randomly, the nuclei will end up mixed.
The result is nuclear fusion, where the two individual atoms are replaced by one larger atom. If the atoms are lighter than the element iron, the resulting fusion will release energy. Through this process, all stars, including our Sun, are powered.

